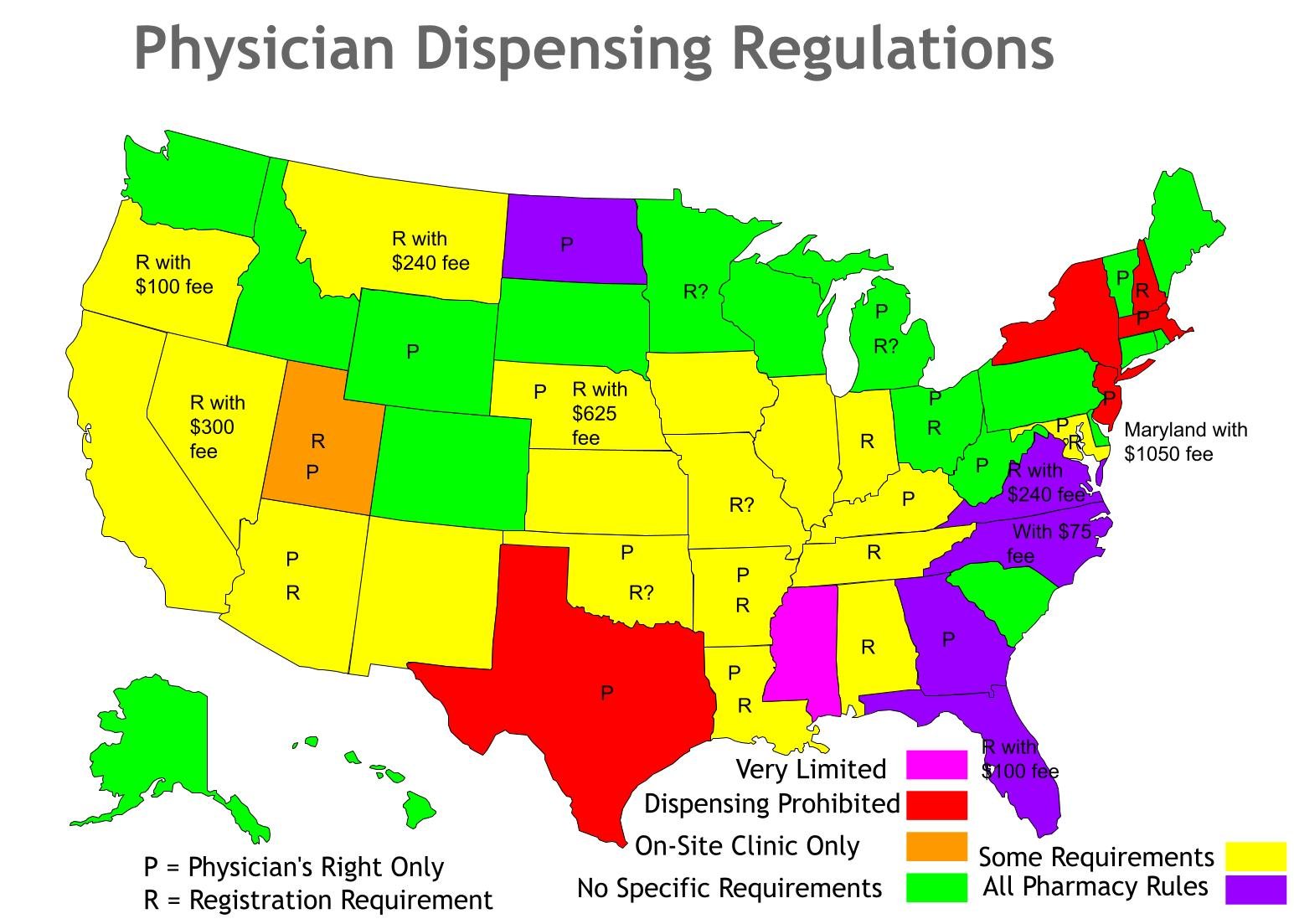
Physician Dispensing State by State Comparison
Dispensing regulations Overview
Please start by reviewing this excellent 2014 overview entitled National Evaluation of Prescriber Drug Dispensing (page 6 contains the most useful chart). Here is a 2011 Physician Dispensing Survey by the National Association of Boards of Pharmacy. This DEA summary contains midlevel practicioner dispensing authorizations by state (thank you to Kathrine S. Nicol of Nicol Health Law for this reference!)
When dispensing please note whether the medication is available over the counter (rather than prescription only). In most states OTC medications are subject to sales tax. In most states prescription only medications are not subject to sales tax (except Illinois and Louisiana).
Packaging - Note that if medications are purchased from a wholesaler they might arrive in packaging that is not designed to be child resistant. These tablets will need to be repackaged in another bottle with a tamper resistant lid unless the patient makes a request to have the medication provided in a standard container. The U.S. Consumer Product Safety Commission (CPSC) administers the Poison Prevention Packaging Act of 1970 (PPPA), 15 U.S.C. §§ 1471-1476. The PPPA requires special (child-resistant and adult-friendly) packaging of a wide range of hazardous household products including most oral prescription drugs. Section 4(b) of the PPPA addresses the need for facilitating access to prescription drugs by elderly and handicapped individuals who have difficulty using special packaging. This guide published by the US Consumer Product Safety Commission is worth reviewing.
"In the case of a household substance which is subject to such a [PPPA] standard and which is dispensed pursuant to an order of a physician, dentist, or other licensed medical practitioner authorized to prescribe, such substance may be dispensed in noncomplying packages only when directed in such order or when requested by the purchaser.”
Please note “CHAPTER III Responsibilities Under the Act – Frequently Asked Questions” which include the following:
“Q. I know of several physicians who dispense prescription drugs for a fee. Are they subject to the provisions of the PPPA?
A. Yes. Physicians who dispense drugs (including drug samples), are, and always have been, subject to the regulations under the PPPA. It is important to note, however, that for the purpose of accommodating elderly and disabled consumers who have difficulty using special packaging, Section 4(b) of the PPPA gives medical practitioners the authority to specify conventional packaging for drugs they prescribe.”
“Q. May an individual request that all of his/her prescriptions be filled in conventional (nonspecial) packaging?
A. Yes, the law does not preclude a pharmacist from relying upon a specific request from a patient to have all of his/her medications placed in non-special packaging. Many pharmacies choose to have this request in writing, i.e., a blanket waiver. However, a single request from a patient to dispense a specific prescription in non-special packaging is not a basis for the pharmacist to infer the patient wants all subsequent prescriptions to be dispensed in non-special packaging. Such a request is not a blanket waiver. A patient who previously requested blanket non-special packaging may later change his/her mind about the use of such packaging because of changing personal circumstances, but may not remember to inform the pharmacist of the change in packaging preference. It is a prudent practice for the dispensing pharmacist to periodically check with all patients who have blanket waiver requests on file to ensure that noncomplying packaging continues to be the preferred packaging choice for the patients' prescription drugs.”
“Q. Must the customer make the choice for conventional packaging in writing?
A. Although many pharmacists do require a written waiver, the law and regulations do not require a written request. The CPSC staff recommends, however, that the pharmacist get a request in writing particularly when a blanket waiver is being requested. This will assist the pharmacist during inspections of the pharmacy by regulatory agencies.”
Dispensing Restrictions and Regulations Vary Widely by State: AZ, AR, GA, KY, LA, MD, MA, MI, NE, NJ, ND, OH, OK, TX, UT, VT, WV, WY are the eighteen states that restrict dispensing to MD, DO, DDS, DPM, DVMs (NPs and PAs will likely not be permitted to dispense in these states). Please verify this information independently and let me know if you spot any inaccuracies.
Physicians planning to draft their own policies about dispensing should consider the helpful guidance offered in Georgia (discussed below).
Alabama - The Alabama Board of Medical Examiners does require that dispensing physicians register. The one page form is not much of a burden and there appears to be no cost associated with the registration. Only those dispensing "controlled substances" need to register, so the board's definition of a controlled substance might be worth clarifying prior to registration.
Physician Dispensing Defined
Physician Dispensing Overview Page
Dispensing Physician's Registration Form
Alaska - They do not have much guidance. The medical board has issued this statement.
Arizona - Physician Dispensing is permitted in Arizona. Physicians do need to register with their appropriate board of medicine. DOs may use this form and MDs may use this form. This DO FAQ form might also be a useful reference.
Arkansas - The in-office dispensing of medications can be more difficult in Arkansas compared to other states. A permit must be obtained from the state medical board. NPs and PAs are not permitted to dispense medications.
California - Physician dispensing is permitted. This practice was challenged and upheld in Park Medical Pharmacy v. San Diego Orthopedic Associates Medical Group, Slip Op. No. D038051 (June 11, 2002), 2002 Cal App 4225. Please see the case above and the California page for a detailed discussion.
Colorado - Permitted. No registration requirement.
Connecticut - Permitted. No registration requirement.
Delaware - Permitted. No registration requirement.
DC - Appears to be permitted without any registration requirement.
Florida - Physician Dispensing is permitted in Florida. They do have a formalized process where the physician must complete the simple three page dispensing form listed at the top of this page and pay a $100 fee. Their detailed description of their dispensing rules can be found on this page. If you would like to dispense medications you should register as a "dispensing practitioner" with the Florida Board of Medicine. Some of the most important language is outlined on the Florida page.
Georgia - Dispensing medications is permitted in Georgia and physicians that wish to dispense must notify the Georgia Composite Medical Board. The Georgia Department of Public Health does have a recommended Drug Dispensing Procedure. The law is cited below, but this set of frequently asked questions from the Drug and Narcotics Agency of the State of Georgia is especially helpful. The law is a good example of clear guidance for physicians, and might be used by physicians in other states with little guidance if they are looking for a reference to develop their own policies and procedures around dispensing. It is discussed in more detail on the Georgia page.
Hawaii - Permitted. No known registration requirement.
Idaho - Permitted. No known registration requirement.
Illinois - Permitted as described here. This application may be used for scheduled drugs, but it is not a customized application for dispensing per se. “Any person licensed under this Act to practice medicine in all of its branches shall be authorized to purchase legend drugs requiring an order of a person authorized to prescribe drugs, and to dispense such legend drugs in the regular course of practicing medicine…”
Indiana - Permitted. Please see Indiana Pharmacy Laws. IC 35-48-7-5.8 a practitioner is defined as, “…a Physician, Dentist, Veterinarians, Podiatrists, Nurse Practitioners, Scientific Investigators, Pharmacists, Hospital, or any other institution or individual licensed, registered, or otherwise permitted to distribute, dispense or conduct research with respect to, or administer a controlled substance in the course of professional practice or research in the United States.”
The Medical Licensing Board of Indiana does not appear to have a formal dispensing physician enrollment process, but contacting them to confirm this may be wise. I do not recommend contacting the Indiana Board of Pharmacy since questioners have reported inconsistent answers from their office.
IC 16-42-3-6 Drugs Dispensed on Prescription appears to be the most relevant section of the Indiana Code to physician dispensing questions and Northwind Pharmaceuticals offers a helpful discussion as well.
Iowa - Permitted. Please see GENERAL PROVISIONS, HEALTH-RELATED PROFESSIONS, §147.107 of the Iowa Code. “A person, other than a pharmacist, physician, dentist, podiatric physician, prescribing psychologist, or veterinarian who dispenses as an incident to the practice of the practitioner’s profession, shall not dispense prescription drugs or controlled substances.” An NP or PA is not permitted to dispense in Iowa.
“A dentist, physician, podiatric physician, or prescribing psychologist who dispenses prescription drugs, other than drug samples, pursuant to this subsection, shall report the fact that they dispense prescription drugs with the practitioner’s respective board at least biennially and shall offer to provide the patient with a written prescription that may be dispensed from a pharmacy of the patient’s choice or offer to transmit the prescription orally, electronically, or by facsimile in accordance with section 155A.27 to a pharmacy of the patient’s choice.”
Kansas - According to this FAQ page: “Physicians should be familiar with the Board’s requirements for dispensing physicians in K.A.R. 100-21-1 through K.A.R. 100-21-5 (found on page 231 and 232). If controlled substances will be dispensed, physicians should be familiar with state pharmacy laws and federal laws which may be applicable.” PAs in Kansas technically may dispense medications under the supervision of a physician, but they are limited to a three day supply. “Pursuant to K.S.A. 65-28a08(b)(2), a PA may dispense prescription-only medications if authorized by their supervising physician. The dispensing of the medications must be in the best interests of the patient and may only be done when pharmacy services are not readily available. Additionally, only a 72-hour supply may be dispensed.”
Kentucky - Permitted. According to KRS 217.182 Sale, distribution, administration, prescription, or possession of legend drugs: “A duly licensed manufacturer, distributor, or wholesaler may sell or distribute a legend drug to… a practitioner” and “[a] practitioner may: (a) Administer, dispense, or prescribe a legend drug for a legitimate medical purpose and in the course of professional practice; or (b) Distribute a legend drug to a person licensed to administer, dispense, distribute, or possess a legend drug. Please see pages 75 and 76 of this detailed KMA Legal Handbook. If a scheduled medication is dispensed this must be reported to KASPER and separate regulations apply (also summarized by the KMA here).
Louisiana - Permitted with medical board registration. Title 46 Professional and Occupational Standards Part XLV. Medical Professions Subpart 3. Practice, Chapter 65. Dispensation of Medication Subchapter C. Registration §6513. Eligibility for Registration as a Dispensing Physician A. “To be eligible for registration as a dispensing physician for all medication, including but not limited to controlled substances and drugs of concern, a physician shall, as of the date of the application: 1. possess a current, unrestricted license to practice medicine duly issued by the board; 2. have successfully completed a graduate medical education training program approved by the board; 3. successfully complete on-line or other training offered by the board respecting its dispensing rules; and 4. not be deemed ineligible for registration as a dispensing physician for any of the causes set forth in §6513.B-D of this Section.” The full code (in Microsoft Word format) can be downloaded on this page by clicking on the link “Title 46:XLV, Professional and Occupational Standards: Medical Professions.” Page 182 of the Word file describes Chapter 65 Dispensation of Medications Subchapter C. Registration §6513. Eligibility for Registration as a Dispensing Physician. This dispensing permit page provides links to the two page application, explains eligibility and discusses the $75 fee.
Maine - Permitted. No registration requirement.
Maryland - For a Maryland physician to dispense medications the physician must submit a detailed application available via email on this page to the Maryland Board of Physicians and include a $1,050 fee. A litany of requirements must be met (some are listed at the bottom of this page). Customized continuing education related to dispensing is required (here is a group of them available from the Maryland State Medical Society). One must submit to inspections and submit information to the board. Dispensing must be "in the public interest" - which in the board's eyes means that a pharmacy is not conveniently available to the patient.
"Licensed dentists, physicians, and podiatrists are required to obtain a dispensing permit if they dispense prescription drugs to patients under their direct care who have informed the provider that a pharmacy is not conveniently available. The licensee shall maintain documentation that should include a single form in each patient's chart for each patient to whom prescription drugs are dispensed. At a minimum, the form shall:
(1) Indicate the reason, as stated by the patient, that a pharmacy is not conveniently available to that patient;
(2) Include a statement signed by the patient indicating that the patient understands that the determination that a pharmacy is not conveniently available is made solely by the patient; and
(3) Be signed and dated by the patient before dispensing prescription drugs to the patient for the first time. See COMAR 10.13.01.04."
Massachusetts - Prohibited. See General Laws Part I, Title XV, Chapter 94C, Section 9
Notwithstanding section 17, a physician… may “dispense by delivering to an ultimate user a controlled substance in a single dose or in a quantity that is, in the opinion of such physician… essential for the treatment of a patient. The amount or quantity of any controlled substance dispensed under this subsection shall not exceed the quantity of a controlled substance necessary for the immediate and proper treatment of the patient until it is possible for the patient to have a prescription filled by a pharmacy. All controlled substances required by the patient as part of the patient's treatment shall be dispensed by prescription to the ultimate user in accordance with this chapter.
This section shall not prohibit or limit the dispensing of a prescription medication that is classified by the department as schedule VI and that is provided by the manufacturer as part of an indigent patient program or for use as samples if the prescription medication is: (i) dispensed to the patient by a professional authorized to dispense controlled substances pursuant to this section; (ii) dispensed in the package provided by the manufacturer; and (iii) provided at no charge to the patient. The department shall promulgate rules and regulations governing the dispensing of medication pursuant to this section. These rules and regulations shall include, but not be limited to, the types and amounts of medications that may be dispensed and the appropriate safeguards for the labeling and dispensing of such medications.
Read page 35 and 36 of the Commonwealth of Massachusetts Board of Registration in Medicine Prescribing Practices Policy and Guidelines Policy 15-05 Adopted October 8, 2015 “Massachusetts physicians are permitted to dispense up to a 30-day supply of Schedule VI sample medications. Physicians may dispense larger supplies of sample medications, up to 90 days, as part of a manufacturer's indigent drug program. All sample medications dispensed to patients, including those provided as part of an indigent patient drug program, must be labeled.”
Michigan - Permitted. No registration requirement. Physicians, NPs and PAs are all permitted to dispense medications in Michigan as discussed in MCL - Section 333.17745.
Minnesota - Permitted. No registration requirement.
Mississippi - Scheduled drug dispensing requirements are understandably extensive but we have not located any registration requirement for dispensing non-scheduled drugs. MS Code § 41-29-305 (2016) states that a “physician or a dentist, in good faith and in the course of his professional practice only, may prescribe, administer, dispense, mix or otherwise prepare narcotic drugs, or he may cause the same to be administered by a nurse or interne under his direction and supervision.” According to Part 2640: Prescribing, Administering and Dispensing: “Dispensing Physician” means any physician who dispenses to a patient for the patient's use any controlled substance, legend drug or other medication where such medication is purchased by the physician for resale to a patient whether or not a separate charge is made. As stated in Part 2617, it is understood that Physician Assistants may not dispense medications.
Source: Miss. Code Ann. §73-43-11 (1972, as amended).
Rule 1.9 Labeling Requirements for Dispensing Physicians. For the purposes of this rule, a “dispensing physician” means any physician who dispenses to a patient for the patient's use any controlled substance, legend drug or other medication where such medication is purchased by the physician for resale to a patient whether or not a separate charge is made. Every dispensing physician, as defined above, who dispenses a controlled substance, legend drug or any other medication must insure that all such substances dispensed be labeled containing the following information:
A. The name of the patient to whom the medication was dispensed.
B. The date that the medication was dispensed.
C. The name, strength and quantity of the medication.
D. Direction for taking or administering the medication.
E. The name and address of the physician dispensing the medication.
The label required by this rule must be written in legible handwriting or typed and must be permanently affixed to the package or container in which the medication is dispensed. Prepackaged samples or starter packs in their original packages or containers need only have the patient name, date distributed, and physician’s name if the manufacturer’s packaging meets other requirements.
No physician may delegate dispensing authority to another person. A physician must personally dispense the medication. For the purpose of this regulation, “personally dispense” means the physician must actually obtain the medication, prepare, count, place the same into the appropriate container and affix the appropriate label to the container.
Missouri - Dispensing is permitted. See “Rules of Department of Insurance, Financial Institutions and Professional Registration Division 2150—State Board of Registration for the Healing Arts Chapter 5—General Rules.” The physician or PA must be present at the time the medication is dispensed and the patient must have the option to obtain the medication at a pharmacy if desired. There are standard labeling and record keeping requirements as well.
Montana - Thanks to the efforts of Institute for Justice Attorney Josh Windham (the Institute for Justice filed a lawsuit in June 2020 to overturn this unconstitutional law) and Dr. Carol Bridges, Dr. Cara Harrop and Dr. Todd Bergland in May 2021 (in response to litigation from this same group) the state passed SB 374 allowing healthcare providers in Montana to dispense medications directly to their patients. I have quoted some of the most important passages below and bolded a few areas of emphasis.
Now “a medical practitioner may dispense drugs if the practitioner:
(a) registers with the board of pharmacy provided for in 2-15-1733; and
(b) complies with the requirements of this section.
(2) Drugs dispensed by a medical practitioner must be:
(a) dispensed directly by the practitioner at the practitioner's office or place of practice;
(b) dispensed only to the practitioner's own patients; and
(c) necessary in the treatment of the condition for which the practitioner is attending the patient.
(3) Before dispensing a drug, a medical practitioner shall offer to give a patient the prescription in a written, electronic, or facsimile form that the patient may choose to have filled by the practitioner or any pharmacy.
(4) Except as otherwise provided in this section, a medical practitioner:
(a) may dispense only those drugs that the practitioner is allowed to prescribe under the practitioner's scope of practice; and
(b) may not dispense a controlled substance.
(5) A medical practitioner dispensing drugs shall comply with and is subject to the provisions of this part and the provisions of:
(a) Title 37, chapter 7, parts 4, 5, and 15;
(b) Title 50, chapter 31, parts 3 and 5;
(c) the labeling, storage, inspection, and recordkeeping requirements established by the board of pharmacy; and
(d) all applicable federal laws and regulations.
(6) A medical practitioner registering with the board of pharmacy shall pay a fee ($240.00) established by the board by rule. The fee must be paid at the time of registration and on each renewal of the practitioner's license.
(7) Except as provided in subsection (8), a medical practitioner registered with the board of pharmacy may not dispense drugs to an injured worker being treated pursuant to Title 39, chapter 71.
Subject only to 37-2-104, 37-7-401, and 37-7-402, this chapter does not: (1) subject a medical practitioner, as defined in 37-2-101, or a person who is licensed in this state to practice veterinary medicine to inspection by the board, prevent the person from compounding or using drugs, medicines, chemicals, or poisons in the person's practice, or prevent a medical practitioner from furnishing to a patient drugs, medicines, chemicals, or poisons that the person considers proper in the treatment of the patient.”
Prior to these updates in 2021 the Montana Code Annotated (2017) 37-2-104 historically stated that “it is unlawful for a medical practitioner to engage, directly or indirectly, in the dispensing of drugs” except… in an emergency, or “whenever there is no community pharmacy available to the patient,” or “the dispensing of drugs occasionally, but not as a usual course of doing business, by a medical practitioner.”
Nebraska - Physician dispensing is permitted without much hassle if the medications are dispensed to the patient without any charge, but if there is a charge then Nebraska law appears to require physicians to complete a three page pharmacy application and pay $625 to register as a pharmacy. The language (explained below) is unfortunately confusing. It appears that Nurse Practitioners and Physician Assistants may not be allowed to dispense at all, but at a minimum would always need to register (presumably through a different process) even in instances where they were dispensing drugs at no charge to the patient. Most states do not use the “pharmacy” term in such a confusing manner. Typically the act of being a pharmacist means that you are dispensing medications based on prescriptions that you did not write (those that were instead written by other practitioners). In Nebraska they define dispensing practitioners by stating that “Health professionals who dispense and charge for drugs and devices as part of their practice must have a pharmacy license. NOTE: Veterinarians, certified nurse midwives, certified registered nurse anesthetists, nurse practitioners, and physician assistants cannot dispense under this type of license.” This page describes the “Dispensing Practitioner Application Processing And Inspection Information.” Unfortunately this $625 fee is owed on an annual basis each July 1st.
Nebraska Revised Statute 38-2850 states: “As authorized by the Uniform Credentialing Act, the practice of pharmacy may be engaged in by a pharmacist, a pharmacist intern, or a practitioner with a pharmacy license. The practice of pharmacy shall not be construed to include: (1) Practitioners, other than veterinarians, certified nurse midwives, certified registered nurse anesthetists, nurse practitioners, and physician assistants, who dispense drugs or devices as an incident to the practice of their profession, except that if such practitioner engages in dispensing such drugs or devices to his or her patients for which such patients are charged, such practitioner shall obtain a pharmacy license;…
Nevada - Dispensing is permitted, but the DPC office must apply by completing this application, paying a $300 fee to the Nevada State Board of Pharmacy, and submitting to an inspection of the premises prior to dispensing any medications. “You must be the only person who prepares prescriptions for dispensing unless you designate an employee or employees to serve as a dispensing technician. Please see “Licensing Application” tab on the home page for the application for a dispensing technician in training. A minimum of 500 hours is required to a dispensing technician in training. If your dispensing address changes, you will be required to submit a new application before moving and pay the $300.00. The new location will require an inspection.'“ Practitioners who dispense only dangerous drugs (not controlled substances) are exempt from reporting requirements.
There is a Self-Assessment Inspection Process form that should be completed. It states “Each practitioner is required to personally order all medications that they will dispense. The practitioner must personally check in the medications ordered for dispensing. On arrival the practitioner must secure all medications that the practitioner will dispense so that no other person, including other practitioners, have access to the medications the practitioner will dispense under his/her dispensing practitioner registration except as allowed under NAC. All invoices for medications must be invoiced to the practitioner. (NAC 639.745) The practitioner also understands that he/she can only dispense medications that the practitioner prescribes, not medications prescribed by another practitioner.” NP and PA dispensing applications are linked on this page.
New Hampshire - Physician dispensing here is essentially prohibited. They are limited to providing a three day supply of medications for the “immediate needs” of the patient. “72 hours: In the ambulatory patient treatment areas of an institution, a medical practitioner may dispense drugs for the immediate needs of the patient but not to exceed a 72-hour supply and only if permitted by the institution.” Please see the New Hampshire Pharmacy Laws & Rules Section 318:42 Dealing in or Possessing Prescription Drugs which states (on page 30 and 31) that “[i]t shall be unlawful for any person who is not a licensed pharmacist in a pharmacy registered in accordance with the provisions of this chapter to manufacture, compound, dispense, sell, offer for sale or have in possession any prescription drug as defined in RSA 318:1, XVII, provided that this section shall not prevent the following: Physicians, dentists, optometrists, podiatrists, veterinarians, advanced practice registered nurses, naturopathic doctors, and physician assistants from possessing, compounding in accordance with RSA 318:14-a, personally administering, or distributing prescription drugs to meet the immediate medical needs of their patients. For advanced practice registered nurses and physician assistants, compounding shall be limited according to RSA 318:42, VIII.”
New Jersey - NJ Rev Stat § 45:9-22.11 makes it clear that "A physician shall not dispense more than a seven-day supply of drugs or medicines to any patient. The drugs or medicines shall be dispensed at or below the cost the physician has paid for the particular drug or medicine, plus an administrative cost not to exceed 10% of the cost of the drug or medicine." There are exceptions if you practice any of the following settings "hospital emergency room, a student health center at an institution of higher education, or a publicly subsidized community health center, family planning clinic or prenatal clinic."
New Mexico - Medication Dispensing Issues: Section 16.10.16.9 DISTRIBUTION OF MEDICATIONS Section A makes it clear that a PA generally may not dispense medications. Section B contains some confusing language: "Distribution of a medication shall be restricted to medications repackaged by a licensed pharmacist or a pharmaceutical manufacturer or re-packager. Physician assistants may request, receive and sign for professional sample medications and may distribute sample medications to patients. A log must be kept of distributed medications in accordance with board of pharmacy regulations. Samples requested/received would be appropriate to the scope of the supervising physician's practice and would be consistent with board of pharmacy regulations." My interpretation: You may dispense medications from wholesalers to your own patients as long as you carefully log them and make no attempt to repackage (count) them in any way. Questions may be directed to the New Mexico Medical Board.
New York - NY - Education Law, Article 137, Pharmacy §6807. Exempt persons. "No prescriber…, may dispense more than a seventy-two hour supply of drugs, except for:
- persons practicing in hospitals as defined in section twenty-eight hundred one of the public health law;
- the dispensing of drugs at no charge to their patients;
- persons whose practices are situated ten miles or more from a registered pharmacy;
- the dispensing of drugs in a clinic, infirmary or health service that is operated by or affiliated with a post-secondary institution;
- persons licensed pursuant to article one hundred thirty-five of this title;
- the dispensing of drugs in a medical emergency as defined in subdivision six of section sixty-eight hundred ten of this article;
- the dispensing of drugs that are diluted, reconstituted or compounded by a prescriber;
- the dispensing of allergenic extracts; or
- the dispensing of drugs pursuant to an oncological or AIDS protocol."
North Carolina - While North Carolina has historically made it difficult to conduct in-office dispensing, a review of their updated rules demonstrates few restrictions, but you will need to register as a dispensing physician and a review of the NC Board of Pharmacy's FAQs will also be helpful. There is an annual $75 fee that is paid to the board of pharmacy.
North Dakota - In 2015 Mark J Hardy, Pharm D, Executive Director of the ND State Board of Pharmacy published this helpful Administrative Guidelines for Practitioner Dispensing in North Dakota. See the North Dakota page for a discussion of requirements, which include a discussion of detailed record keeping rules, etc.
Ohio - Permitted. No registration requirement. In office dispensing is permitted in Ohio. The State of Ohio Board of Pharmacy requires that dispensing physicians obtain a Terminal Distributor of Dangerous Drugs license. All the details and applications can be found on this Ohio Board of Pharmacy website.
Oklahoma - Physician dispensing is permitted, even via machine, but not specifically discussed in the medical practice act. The Oklahoma Board of Medical Licensure and Supervision has lots of helpful forms available on this page. A variety of policies including a medical office audit form and a policy on internet prescribing.
Okla. Admin. Code § 435:15-11-1 Prescriptive and dispensing authority (h) makes it clear that PAs may not dispense medications. "Physician Assistants may not dispense drugs, but may request, receive and sign for professional samples and may distribute professional samples directly to patients in accordance with written policies established by the supervising physician."
A medical board newsletter states "PAs and NPs cannot dispense drugs but may distribute samples" but it does not cite the Oklahoma Administrative Code, so it remains unclear whether the board of nursing agrees that NPs are not permitted to dispense.
Oregon - The Oregon Board of Pharmacy issued a Permanent Administrative order in Dec 2017 (BP 4-2017, CHAPTER 855, BOARD OF PHARMACY) rule 855-043-0505 “Dispensing Practitioner Drug Outlets.” “A practitioner's facility that engages in dispensing FDA-approved human prescription drug therapies greater than a 72 hours supply or any medication refill must register their dispensing site as a drug outlet with the Board as a DPDO on a form provided by the Board, and must renew its registration annually on a renewal form provided by the Board.” An initial application must be accompanied by the fee established in division 110 of this chapter ($100 annually expiring each March 31). The registered DPDO must maintain written policies and procedures for the management of drugs intended for dispensing, to include security, acquisition, storage, dispensing and drug delivery, disposal and record keeping (for at least three years).
Pennsylvania - Permitted. No registration requirement. The 2013 PA House Bill No. 1846 has been a source of the confusion for some. The thirty-day limit described in this bill applies when the patient is seen pursuant to workers comp regulations. DPC practice dispensing is generally outside of workers comp program, thus the 30 day limit typically would not apply.
PA Code Title 28 § 25.95. Mandatory compliances.
Any practitioner who is registered or licensed by the appropriate State Board to dispense drugs to patients is required to comply with § § 25.93 and 25.94 (relating to labeling—drug code number; and expiration date of drug).
§ 25.93. Labeling—drug code number.
The label on a dispensed drug container shall include the name of the drug, using abbreviations if necessary, the quantity, and the name of the manufacturer if the drug is a ‘‘generic’’ drug. In those situations where a practitioner specifically indicates that the name of the drug should not appear on the label, the recognized national drug code number should be placed on the label if such a number is available for the product. When a drug is dispensed by a practitioner other than a pharmacist, the label shall also bear the name and address of the practitioner, the date dispensed, the name of the patient, and the directions for the use of the drug by the patient.
§ 25.94. Expiration date of drug.
Drugs which at the time of their dispensing have full potency for less than one year, as determined by the expiration date placed on the original label by the manufacturer, may only be dispensed by a practitioner with a label that indicates said expiration date. The label should include the statement: ‘‘Do not use after (manufacturer’s expiration date)’’ or similar wording.
PA Code Title 28 § 25.114. Persons exempt from registration. (3) Practitioners licensed by law to prescribe, administer or dispense drugs or devices when operating under the authority of the licensure. Registration is required if practitioners engage in the manufacture or distribution of drugs or devices.
The language of 49 Pa. Code § 18.158 describes dispensing for Physician Assistants. It does not appear to restrict them to any specific number of days. It does state that they may not prescribe more than 30 days’ worth of a schedule 2 controlled substance.
Rhode Island - Appears to be permitted without any registration requirement.
South Carolina - Permitted. No registration requirement.
South Dakota - Permitted. No registration requirement.
Tennessee - Permitted under the Rules of the Tennessee Board of Medical Examiners see Section 0880-2-.14 Specially Regulated Areas and Aspects of Medical Practice Pages 38-46. “Physicians who elect to dispense medication for remuneration must comply with the following: (a) All Federal Regulations (21 CFR 1304 through 1308) for the dispensing of controlled substances. (b) Requirements for dispensing of non-controlled drugs are as follows:
“1. Drugs are to be dispensed in an appropriate container labeled with at least, the following: (i) Patient’s name. (ii) Date. (iii) Complete directions for usage. (iv) The physician’s name and address (v) A unique number, or the name and strength of the medication. 2. Physicians may dispense only to individuals with whom they have established a physician/patient relationship. It shall be a violation of this rule for a physician to dispense medication at the order of any other physician not registered to practice at the same location. 3. Whenever dispensing takes place, appropriate records shall be maintained. A separate log must be maintained for controlled substances dispensing.”
Texas - For those seeking additional information about in office dispensing hurdles, please review the text of the Texas Occupations Code, Title 3 Health Professions, Subtitle B Physicians, Chapter 158 Authority of Physician to Provide Certain Drugs and Supplies. The problem is also discussed on pages 6 & 7 of this review manuscript on dispensing. Attempts have been made to correct this law, but the pharmacy lobby has repeatedly opposed any updates.
Fortunately Michael Garrett, MD (a DPC Physician) and Kris Held, MD (an ophthalmologist) have joined up with the Institute for Justice to make an effective state constitutional law argument against the state boards of both medicine and pharmacy. Here is a copy of the complaint that was filed in June 2019.
Utah - Historically the state had a total prohibition of in office physician dispensing. Modifications occurred in 2014 and 2015. As described in Utah Code Section 58-17b-801 to 58-17b-806 all physicians are now permitted to submit an application for a license as a "dispensing medical practitioner." These code sections are now part of the entire Utah Pharmacy Practice Act. If the license is obtained the physician will now be able to dispense only "cosmetic drugs," "injectable weight loss drugs," or a "cancer drug treatment regimen." These exceptions do not do much for most DPC practices. On-site "employer sponsored clinics" receive favorable treatment and are permitted to dispense routine medications that are "prepackaged drugs" that are provided "in a fixed quantity per package by a pharmaceutical wholesaler or distributor." If you want to open a DPC practice in Utah where you can dispense medications, then you need to do it as an onsite clinic treating only employees (not open to the general public) and you need to be able to dispense the medications without the use of a pill counter (which obviously requires repackaging - a prohibited move). For those that which to prescribe controlled substances (not recommended) additional rules apply. Here is the link to the Dispensing Medical Practitioner application. Here is a link to the Dispensing Medical Practitioner Clinic application.
Vermont - Permitted. No registration requirement.
Virginia - DOs and MDs may dispense if they are licensed by the Virginia Board of Pharmacy. For details please see this FAQ page or download this Guidance Document 110-29. This link with download the entire regulatory document. Here are additional links:
Application for a License to Sell Controlled Substances (usually $180 or $240)
Practitioner Inspection Report
Facility Permit Application
Washington - Permitted. No registration requirement.
West Virginia - Permitted. No registration requirement.
Wisconsin - Permitted. No registration requirement. Physicians and PAs are allowed to dispense medications. NPs are not permitted to dispense medications.
Wyoming - Permitted. No registration requirement.